- Home
-
![]()
 You are here: Home » Blogs » Oral Care Guidebook » Miswak Toothpaste Tablets: Technical Trends & OEM Development Insights for 2025
You are here: Home » Blogs » Oral Care Guidebook » Miswak Toothpaste Tablets: Technical Trends & OEM Development Insights for 2025Miswak Toothpaste Tablets: Technical Trends & OEM Development Insights for 2025
Views: 0 Author: Site Editor Publish Time: 2025-11-27 Origin: Site
Intro: 2025 Is the Industrial Turning Point for Miswak in Waterless Oral Care
By 2025, the competition in waterless toothpaste tablets has shifted from “eco-friendly concept” to true industrial scalability. Brands are no longer satisfied with generic mint tablets—they are actively looking for ingredients that represent cultural value, natural care, and scientific credibility.
This is exactly why Miswak (Salvadora persica) is rising again as a global formulation focus.However, making a stable Miswak toothpaste tablet is far beyond simply adding Miswak powder to a base formula. The powder behavior, tablet structure, disintegration system, flavor engineering, active stability, and batch consistency determine whether a product is ready for mass production and long-term commercialization.
Based on over a decade of experience in OEM/ODM waterless oral-care manufacturing, this article outlines the real technical challenges and industrial trends of Miswak toothpaste tablets in 2025.
1. What the Market Cannot See: The Real Engineering Challenges of Miswak in Solid Formulation
Most public articles talk about the cultural and natural benefits of Miswak.But engineers know the real difficulty lies in how Miswak behaves as a powder inside a compressed solid dosage form.
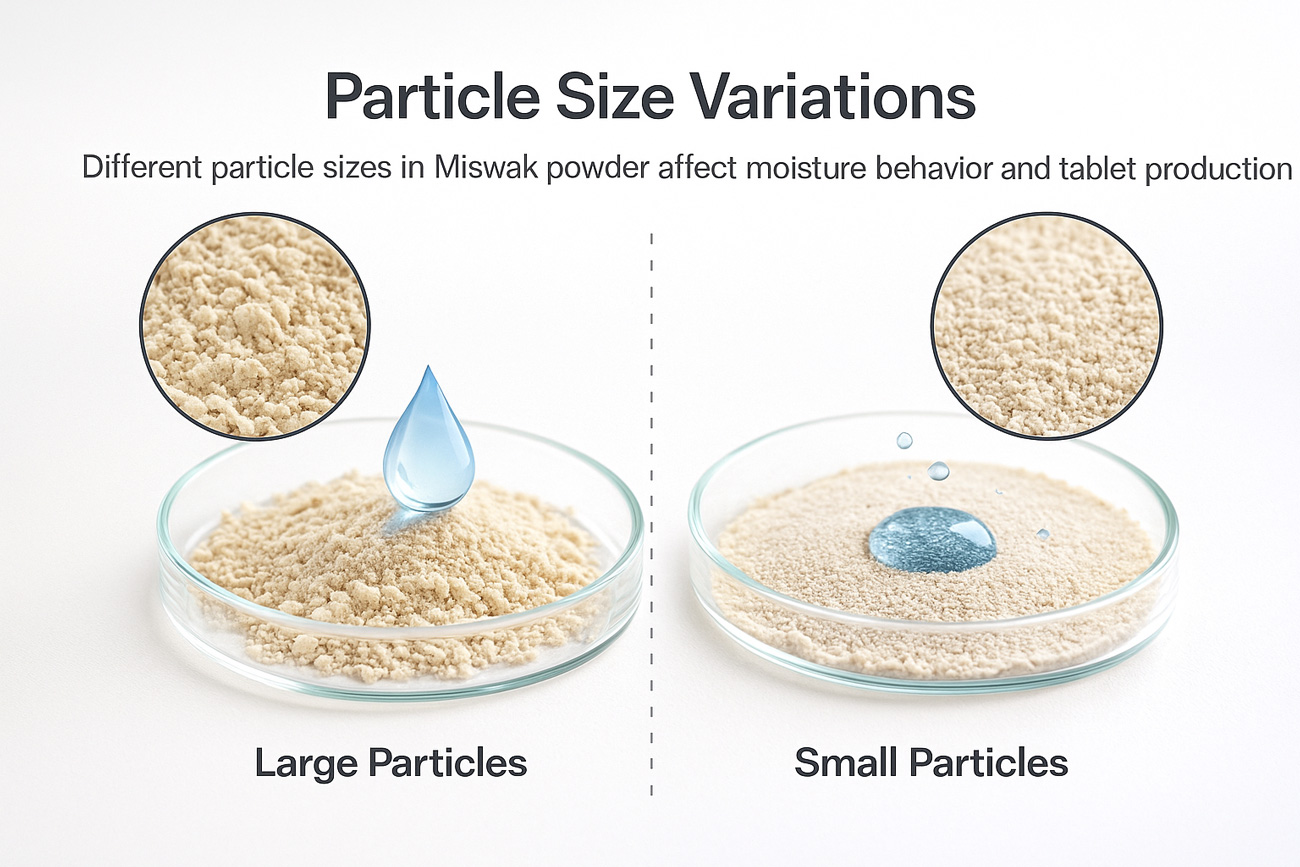
1.1 Irregular Particle Size Directly Affects Tablet Structure
Common Miswak powder issues:
High fiber content
Wide particle size distribution
Batch-to-batch moisture variation
Noticeable color differences
Possible issues in tablets:
Coarse particles → unstable disintegration
Over-fine particles → fragile tablets and poor hardness
High moisture → weak compaction and higher water activity
Color variation → inconsistent appearance between batches
Therefore, the first step in Miswak formulation is not the percentage—it is:
✔ Powder classification
✔ Standardizing particle size
✔ Moisture & water-activity controlThis step alone separates experienced factories from inexperienced ones.
1.2 High Friction and Poor Flowability: The Hidden Problem of Natural Powders
Miswak’s natural fibers cause a significantly higher friction coefficient, leading to:
Uneven feeding → fluctuating tablet weight
Faster tooling wear
Uneven pressure distribution → internal voids or cracks
The engineering solutions include:
Optimizing lubricant systems
Adjusting excipient ratios
Using low-friction tooling
Applying flow-enhancing functional excipients developed specifically for solid oral-care
These are practical, machine-level optimizations—not theoretical assumptions.
1.3 Miswak Interferes With Disintegration — Engineering Compensation Is Required
Plant fibers naturally slow disintegration.
This means the upper limit of Miswak addition is determined not by marketing, but by:Disintegrant gradient testing
Particle-size combinations
Tablet density management
Pressure curve adjustments
A professionally tuned formula should achieve 15–25 seconds of consistent disintegration, even at scale.
![Miswak Particle Size Variations Miswak Particle Size Variations]()
2. Active Stability: The Most Overlooked but Most Critical Topic for 2025
Unlike synthetic actives, Miswak contains heat-sensitive and oxidation-sensitive components. Stability is influenced by temperature, oxygen, water activity, light exposure, and processing time.
2.1 Water Activity (Aw) Control
If the raw material's Aw is too high, issues may include:
Faster degradation of natural actives
Risk of moisture absorption in finished tablets
Dull or muted flavor
Increased microbiological risk
A mature OEM will perform:
Incoming water-activity testing
Drying or conditioning when needed
Secondary testing before mixing
2.2 Protecting Miswak’s Actives During Processing
High-speed mixing can cause temperature rise, which accelerates degradation.
Engineering controls include:Low-temperature mixing
Stepwise mixing programs
Paddle types that reduce shear stress on plant powders
These procedures are specific to solid oral-care—not borrowed from toothpaste manufacturing.
2.3 Preventing Oxidation & Color Shift
Miswak contains polyphenols, which oxidize easily.
To maintain color and activity:Add natural antioxidant systems
Reduce oxygen exposure during packaging
Conduct light-stability evaluations
Implement a measurable color-difference management system
This is key for ensuring both appearance and functional stability in shelf-life tests.
3. Industrial-Scale Manufacturing: Consistency Is the Real Barrier
The hardest part of Miswak toothpaste tablets is not making a prototype—it is producing 10,000–100,000 bottles consistently.
3.1 Color Variation: The Constant Challenge of Natural Powders
Plant-based ingredients vary with:
Harvest season
Climate
Extraction conditions
Professional factories adopt:
Colorimeter standards
Color-grading cards
Defined acceptable deviation thresholds
Uniform mixing procedures
Color-compensation strategies when required
This avoids visible shade differences across batches.
3.2 Tablet Weight Stability Determines User Experience
Weight variation affects:
Disintegration
Mouthfeel and cleaning performance
Active-ingredient delivery
A robust system includes:
Stable feeding equipment
Bulk-density management
Pressure compensation adjustments
Automatic weight monitoring
Industry expectation: ±2% tablet weight variation.
3.3 Packaging Has a Direct Impact on Product Stability
Common packaging types:
Glass jars (best stability)
Aluminum tins
Paper pouches (require strict moisture & oxygen protections)
Each packaging requires full stability validation:
Moisture-ingress testing
Light-exposure testing
Seal-integrity checks
Transportation vibration assessments
This step is often ignored by small manufacturers but essential for export-ready products.
4. Flavor Engineering: The Decisive Factor for Consumer Acceptance
Miswak has a natural herbal, slightly astringent note. If not properly balanced, consumers may reject the product on first use.
4.1 Balancing Herbal Notes With Modern Freshness
Critical considerations:
Too strong cooling agents → overwhelm Miswak’s identity
Too strong herbal notes → increase bitterness/astringency
A professional flavor system requires:
Blending different mint oils
Adjusting sweetness gradients
Controlling cooling-agent release curves
The goal: clean, refreshing, yet naturally herbal.
4.2 Flavor Must Be Compatible With Tablet Compression
Solid-form flavors differ from toothpaste or mouthwash flavors. They must:
Resist pressure
Avoid absorbing moisture
Maintain integrity during mixing
Provide stable, gradual release
This is a key threshold for true waterless oral-care formulation capability.
5. OEM Trends for Miswak Toothpaste Tablets in 2025
Based on recent development requests and market launches, three clear trends are emerging.
Trend 1 — Miswak Is Moving From “Supporting Ingredient” to “Core Concept”
Brands now request:
Miswak-centered packaging
Stronger communication around natural herbal cleansing
Full Miswak product lines
It is becoming a mainstream product direction rather than a niche concept.
Trend 2 — Combination Formulas Are Increasing Rapidly
Popular combinations include:
Miswak + Hydroxyapatite
Miswak + Xylitol
Miswak + Papain plant enzyme
The goal is to combine natural identity with modern functionality.
Trend 3 — Flavor Innovation Is Becoming a Differentiation Power
Consumers want:
Gentle herbal freshness
Softer botanical notes
Non-irritating but clean mouthfeel
Brands will compete heavily on flavor innovation.
Conclusion: The Future of Miswak Toothpaste Tablets Belongs to Factories With Real Technical Strength
Miswak is a natural ingredient with complex powder behavior and stability challenges.
To achieve reliable mass production, factories must excel in:Powder-engineering capability
Disintegration-system optimization
Botanical-raw-material handling
Tablet-compression engineering
Flavor-system design
Batch-consistency and stability control
As waterless oral care enters its next stage, brands increasingly need manufacturing partners that can make Miswak products stable, consistent, and scalable—not just functional in prototypes.
Factories with these capabilities will become the true strategic partners in the global expansion of natural solid oral-care products.
Related ProductsProduct Category
Other Links